Very Encouraging Phase 2 PMS Results
NEU released impressive efficacy and safety results today from the first of four single-arm Phase 2 trials evaluating NNZ-2591 (n=18). Results in patients with Phelan-McDermid syndrome (PMS) showed statistically significant and clinically meaningful
improvements in efficacy outcomes, as measured by clinicians and caregivers, and a well-tolerated safety profile. In particular:
- Clinician overall assessment of improvement (CGI-I) mean score of 2.4. We had flagged ~3.0-3.5 would be a positive outcome, hence, this 2.4 is a great result (lower = better). Additionally, 16/18 children had an improvement from baseline, with 10/18 deemed “very much improved” or “much improved”.
- Caregiver overall assessment of improvement (CIC) mean score of 2.7. Similarly, 15/18 children had any degree of improvement from baseline, with 7/18 deemed “very much improved” or “much improved”.
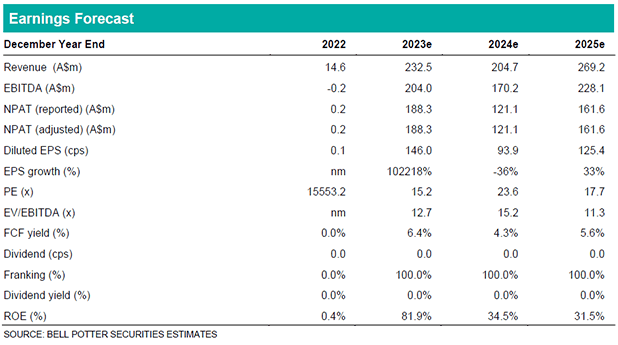
NNZ-2591 was generally well tolerated by the 18 subjects, with only one severe TEAE unrelated to the study drug and low rates of discontinuation. Diarrhea, a common side effect of NEU’s first drug trofinetide, has not presented as an issue for NNZ-2591. Looking ahead, we expect NEU will look to commence a larger placebo-controlled Phase 3 PMS trial to confirm the initial results seen in this single-arm study. NEU have more than enough cash at hand (A$230m as at 30th Sep 2023) to do so.
Investment View: Upgrade to BUY, $27.00 PT
We have far greater confidence in NNZ-2591 following the Phase 2 results and therefore materially increase our Probability of Success in PMS and the other three indications which are similarly characterised disorders. We increase our PT to $27.00 following the increased value attributed to NNZ-2591. This represents >15% upside to the current price hence we upgrade to a BUY. Additional catalysts are expected to continue driving interest over the next 12 months, specifically (1) DAYBUE quarterly updates in the US, (2) DAYBUE submissions in Canada & Europe, (3) additional NNZ-2591 Phase 2 results, and (4) NNZ-2591 Phase 3 preparations.